A high sulfur content composite with core–shell structure as cathode material for Li–S batteries
Abstract
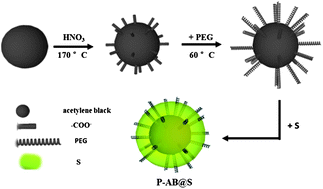
Lithium–sulfur (Li–S) batteries have received significant attention in recent years because of their high theoretical specific capacity (1675 mA h g−1) and energy density (2600 W h kg−1). Many papers focus on cells that exhibit very high capacity per gram of sulfur, which contain sulfur contents well below 50% which greatly reduces their overall energy density per gram of cathode. Moreover, they do not address the issues of practical sulfur loading and large-scale technology for commercial applications. In general, the lower the sulfur content, the higher the sulfur capacity. In this paper, a high sulfur content (80% S) carbon–sulfur (P-AB@S) material with core–shell structure has been successfully synthesized by grafting of

 Please wait while we load your content...
Please wait while we load your content...