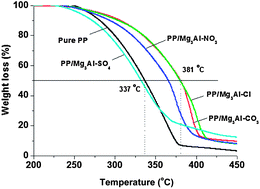
In this contribution, polypropylene (PP) nanocomposites with four different inorganic anion (CO32−, NO3−, Cl−, and SO42−) intercalated Mg3Al layered double hydroxides (LDHs) as nanofillers were prepared using a solvent mixing method for the first time. The influence of interlayer inorganic anions on the thermal stability, melting and recrystallisation behavior and rheological property of PP was then systematically compared. The thermal stability was significantly improved, with Mg3Al–CO3 and Mg3Al–Cl LDHs, and T0.5 increased by about 44 °C compared to pure PP. The incorporation of LDHs increased the melting temperature (Tm) and the recrystallization temperature (Tc) of PP by about 3–4 °C and 10–13 °C, respectively. The influence on the storage modulus (G′) and loss modulus (G′′) follows the order of Mg3Al–SO4 > Mg3Al–NO3 > Mg3Al–CO3 > Mg3Al–Cl. Although the incorporation of all LDHs led to an improvement in the thermal stability, Tm, Tc, G′, G′′, and complex viscosity of PP/Mg3Al–X LDH nanocomposites, the extent of changes varied with the type of inter-layer anions. The study finally demonstrated that even very similar inorganic anions such as CO32−, NO3−, Cl−, and SO42− could lead to a significant difference in the property of the synthesized PP/LDH nanocomposites, and it must be taken into account for the future design of polymer/LDH nanocomposites.

 Please wait while we load your content...
Please wait while we load your content...