Room-temperature synthesis of ZIF-90 nanocrystals and the derived nano-composite membranes for hydrogen separation
Abstract
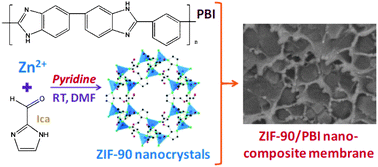
Nanocrystals of ZIF-90 have been synthesized at room temperature through a novel procedure and incorporated into PBI-based nano-composite membranes for hydrogen purification. The physical and chemical structures of the ZIF-90 nanoparticles have been examined via multiple advanced instrumental analyses including DLS, XRD, FESEM, NMR and FTIR. The nanocrystals show identical morphology, crystallinity and chemical structure but a significantly reduced particle size (around 100 nm) when compared with the ZIF-90 particles in previous studies. The derived ZIF-90–PBI nano-composite membranes exhibit homogeneous particle dispersion and fine particle–polymer adhesion, as well as excellent hydrogen purification performance at various testing conditions. The 45/55 (w/w) ZIF-90–PBI membrane with the highest ZIF-90 volume loading of up to 50.9 vol% possesses the best ideal H2–CO2 separation performance with a moderate H2 permeability of 24.5 Barrer and a high H2–CO2 selectivity of 25.0 in pure gas permeation tests at 35 °C. The membrane also shows promoted gas separation performance during mixed gas tests at 180 °C with an H2 permeability of 226.9 Barrer and an H2–CO2 separation factor of 13.3 that surpasses the latest Robeson upper bound for H2–CO2 separation. This work not only expands the field of nano-composite membrane fabrication, but also provides prospects for interdisciplinary research combining nano-science and chemical engineering for clean energy development.

 Please wait while we load your content...
Please wait while we load your content...