Mechanistic study of the electrochemical extraction of K+ from KFeSO4F
Abstract
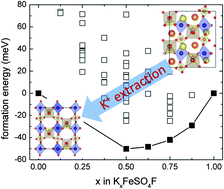
KFeSO4F was recently reported as a cathode candidate for the removal and insertion of Li+, Na+ and K+. Here the mechanism for the electrochemical extraction of K+ from KFeSO4F is unveiled using density functional calculations. Consistent with experimental observations, first-principles calculations predict a thermodynamically favourable phase transition when the potassium content in KxFeSO4F is below 50% (x ≤0.5). The mechanism that drives this phase transition is carefully investigated. It is shown that the extraction of K+ can be expressed as a sequential removal of K+ and selective

 Please wait while we load your content...
Please wait while we load your content...