Hydroxide ion conduction in molybdenum(vi)-doped tin pyrophosphate at intermediate temperatures†
Abstract
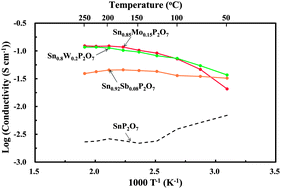
Research efforts focused on alkaline fuel cells have increased with the goal of commercialization. Anion exchange polymers are widely viewed as promising candidates for electrolyte membranes; however, the operating temperature of these polymers is limited to 80 °C or lower. Operation of a fuel cell at elevated temperatures provides the anode catalyst with a high tolerance towards CO and enhances the electrode reaction kinetics. We present a new type of inorganic hydroxide ion-conducting compound that has the potential to meet the demands for intermediate temperature applications. A series of Sn1−xAxP2O7 (AVI = Mo and W) compounds were synthesized, of which Sn0.85Mo0.15P2O7 exhibited the highest electrical conductivity over a wide temperature range (0.02 S cm−1@50 °C, 0.07 S cm−1@100 °C, 0.10 S cm−1@150 °C, and 0.123 S cm−1@200 and 250 °C). Such a high conductivity was also confirmed under conditions of fuel cell operation. The anion exchange capability of this compound was evaluated using spectroscopic and electrochemical analyses, the results of which indicated that hydroxide ions are incorporated into the compound by charge compensation for high valency cations and hydroxide ion conduction is predominant in the temperature range of 50–250 °C.

 Please wait while we load your content...
Please wait while we load your content...