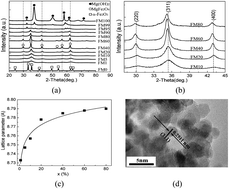
Ultrafine superparamagnetic magnesium ferrite nanocrystallites were successfully synthesized by doping Mg2+ into α-Fe2O3 in a solvent thermal process. The dopant altered the microstructure of the α-Fe2O3 which resulted in an enhanced arsenic adsorption performance and an easy magnetic separation capability. At a concentration of 10%, Mg-doping largely increased the surface area to ∼438 m2 g−1, enhanced the dispersion and contact with arsenic species in water due to its superparamagnetic behavior, and largely improved the arsenic adsorption performance in both lab-prepared and natural water samples at near neutral pH when compared with ultrafine α-Fe2O3 nanoparticles. At an Mg concentration of 10%, the amounts of As(III) and As(V) adsorbed reached 9.3 mg g−1 and 10 mg g−1, respectively, at a low equilibrium arsenic concentration of less than 10 μg L−1. The nanocrystallites could be easily separated from water when an external magnetic field was present after water treatment due to their high saturation magnetization, which solved the potential nanomaterial dispersion problem and facilitated arsenic desorption and reuse of the material.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...