Tuning of the optoelectronic properties of peptide-appended core-substituted naphthalenediimides: the role of self-assembly of two positional isomers†
Abstract
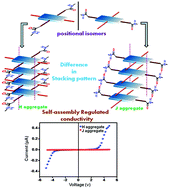
This study demonstrates how the self-assembly pattern of two different and isomeric peptide-appended core-substituted naphthalenediimides (NDIs) affects the modulation of their optoelectronic properties. Two isomeric peptide-attached NDIs were synthesized, purified and characterized. Interchanging the position of attachment of the peptide units and the alkyl chains in the NDI has altered the respective self-assembling patterns of these isomeric molecules in the aggregated states. The isomer having a peptide moiety in the core position and the alkyl chain in the imide position (compound N1) forms face to face stacking or ‘H’ aggregates in aliphatic solvents including n-hexane, and n-decane, whereas compound N2, in which the peptide moiety is at the imide position and the alkyl chain is attached at the core position of NDI exhibits edge to edge stacking or J aggregates under the same conditions as it is evident from their UV-vis studies. The H aggregated species (obtained from N1) show inter-connected nanofibers, whereas the J aggregated species (obtained from N2) exhibit the morphology of helical nanoribbons. FT-IR and X-ray diffraction studies are in favor of the same aggregation behavior. The individual packing patterns of these two peptide-based isomers have a direct impact on their respective electrical conductivity. Interestingly, the H aggregated species shows 100 times greater current conductivity than that of the J aggregate. Moreover, it is only the H aggregated species that exhibits a photocurrent, and no such photocurrent response is observed with the J aggregates. Computational studies also support that different types of aggregation patterns are formed by these two isomeric molecules in the same solvent system. This unique example of tuning of optoelectronic behavior holds future promise for the development of new peptide-conjugated π-functional materials.


 Please wait while we load your content...
Please wait while we load your content...