Overcharging of polyelectrolyte complexes: an entropic phenomenon†
Abstract
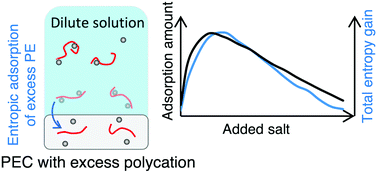
Overcharging in complex coacervation, in which a polyelectrolyte complex coacervate (PEC) initially containing equal moles of the cationic and anionic monomers absorbs a large excess of one type of polyelectrolyte species, is predicted using a recently developed thermodynamic model describing complexation through a combination of reversible ion binding on the chains and long-range electrostatic correlations. We show that overcharging is favored roughly equally by the translational entropy of released counterions and the binding entropy of polyelectrolytes in the polyelectrolyte complex, thus helping resolve competing explanations for overcharging in the literature. We find that the extent of overcharging is non-monotonic in the concentration of added salt and increases with both strength of ion-pairing between polyions and chain hydrophobicity. The predicted extent of overcharging of the PEC is directly compared with that of multilayers made of poly(diallyldimethylammonium), PDADMA, and poly(styrene-sulfonate), PSS, overcompensated by the polycation in two different salts: KBr and NaCl. Accounting for the specificity of salt ion interactions with the polyelectrolytes, we find good qualitative agreement between theory and experiment.


 Please wait while we load your content...
Please wait while we load your content...
