pH-Responsive aggregates transition from spherical micelles to WLMs induced by hydrotropes based on the dynamic imine bond
Abstract
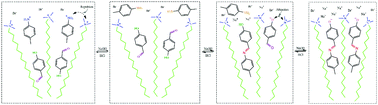
In recent years, the use of dynamic chemical bonds to construct stimulus-responsive micelle systems has received increasing attention. However, current reports focus on the construction of dynamic covalent bond surfactants using dynamic chemical bonds, and the method of applying dynamic covalent bonds to hydrotropes has not been reported yet. In this study, a novel pH-responsive worm-like micelle system was constructed by mixing cetyltrimethylammonium bromide (CTAB), 4-hydroxybenzaldehyde (HB) and p-toluidine (MB) at the molar ratio of 60 mM : 40 mM : 40 mM. The formation mechanism of the dynamic covalent bond hydrotropes and the rheological behavior of the micelles were investigated via rheology, 1H-NMR spectroscopy and Cryo-TEM. The results show that as the pH increases, the viscosity of the solution first decreases and then increases rapidly. The microscopic aggregates in the solution transition from spherical micelles to worm-like micelles (WLMs), and the solution changes from a water-like fluid without viscosity to a gel system that can withstand its own weight. The transformation of the aggregates and their rheology can be attributed to the formation of MB–HB−, which is a type of hydrotrope with dynamic covalent bonds. Moreover, the transition from spherical micelles to worm-like micelles in this system is reversible.


 Please wait while we load your content...
Please wait while we load your content...