Free-energy landscape of polymer-crystal polymorphism†
Abstract
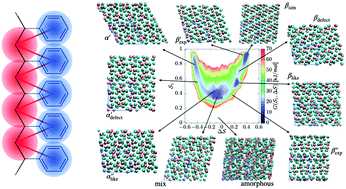
Polymorphism rationalizes how processing can control the final structure of a material. The rugged free-energy landscape and exceedingly slow kinetics in the solid state have so far hampered computational investigations. We report for the first time the free-energy landscape of a polymorphic crystalline polymer, syndiotactic polystyrene. Coarse-grained metadynamics simulations allow us to efficiently sample the landscape at large. The free-energy difference between the two main polymorphs, α and β, is further investigated by quantum-chemical calculations. The results of the two methods are in line with experimental observations: they predict β as the more stable polymorph under standard conditions. Critically, the free-energy landscape suggests how the α polymorph may lead to experimentally observed kinetic traps. The combination of multiscale modeling, enhanced sampling, and quantum-chemical calculations offers an appealing strategy to uncover complex free-energy landscapes with polymorphic behavior.
- This article is part of the themed collection: 2021 Soft Matter Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...