Pearl-necklace assembly of human serum albumin with the poly(acrylic acid) polyelectrolyte investigated using small angle X-ray scattering (SAXS)†
Abstract
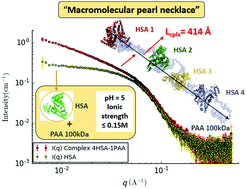
In this comprehensive study, the interaction of human serum albumin (HSA) with poly(acrylic acid) (PAA) was explored using small angle X-ray scattering (SAXS) combined with chromatography. The results revealed the formation of a complex between HSA macromolecules and PAA chains but solely under some specific conditions of the ionic strength and pH of the medium. In fact, this binding was found to take place only at pH close to 5 and at low ionic strength (0.15 M). Otherwise, for a higher pH and a salt concentration of 0.75 M the HSA–PAA complex tends to dissociate completely showing the reversibility of the complexation. The assessment of the influence of the HSA/PAA molar ratio on the radius of gyration of the complex suggests that 4 HSA molecules could bind to each 100 kDa PAA chain. In addition, the Porod volume evaluation for the same range of the HSA/PAA ratio confirms this assumption. Finally, an all-atom SAXS modelling study using the BUNCH program was conducted to find a compatible model that fits the HSA–PAA complex scattering data. This model allows us to portray the HSA/PAA complex as a pearl-necklace assembly with 4 HSA molecules on the 100 kDa PAA chain.


 Please wait while we load your content...
Please wait while we load your content...