Partition of nanoswimmers between two immiscible phases: a soft and penetrable boundary
Abstract
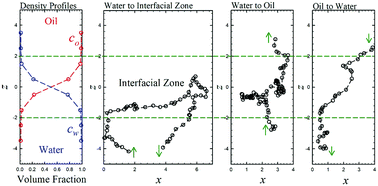
The behavior of run-and-tumble nanoswimmers which can self-propel in two immiscible liquids such as water–oil systems and are able to cross the interface is investigated by dissipative particle dynamics. At the steady-state, the partition ratio (φ) of nanoswimmers between the two immiscible liquids is obtained, and it depends on the active force (Fa), run time (τ), and swimmer–solvent interactions. The partition ratio φ is found to grow generally with increasing Fa2τ. At sufficiently large Fa, it is surprising to find that hydrophilic nanoswimmers prefer to stay in the oil phase rather than in the water phase. The partition ratio is also influenced by the hydrophobicity of swimmers in the oil phase. Two simple models are proposed to describe the partition ratio, including a near-equilibrium model and a kinetic model. Surface accumulation appearing at an impenetrable interface is also observed at the fluid–fluid interface for small Fa but it vanishes for sufficiently large Fa.


 Please wait while we load your content...
Please wait while we load your content...