Assessing the extent of the structural and dynamic modulation of membrane lipids due to pore forming toxins: insights from molecular dynamics simulations†
Abstract
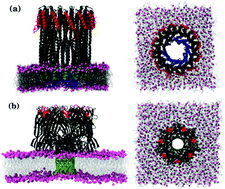
Infections caused by many virulent bacterial strains are triggered by the release of pore forming toxins (PFTs), which form oligomeric transmembrane pore complexes on the target plasma membrane. The spatial extent of the perturbation to the surrounding lipids during pore formation is relatively unexplored. Using all-atom molecular dynamics simulations, we investigate the changes in the structure and dynamics of lipids in a 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) lipid bilayer in the presence of contrasting PFTs. Cytolysin A (ClyA), an α toxin with its inserted wedge shaped bundle of inserted α helices, induces significant asymmetry across the membrane leaflets in comparison with α hemolysin (AHL), a β toxin. Despite the differences in hydrophobic mismatch and uniquely different topologies of the two oligomers, perturbations to lipid order as reflected in the tilt angle and order parameters and membrane thinning are short ranged, lying within ∼2.5 nm from the periphery of either pore complex, and commensurate with distances typically associated with van der Waals forces. In contrast, the spatial extent of perturbations to the lipid dynamics extends outward to at least 4 nm for both proteins, and the continuous survival probabilities reveal the presence of a tightly bound shell of lipids in this region. Displacement probability distributions show long tails and the distinctly non-Gaussian features reflect the induced dynamic heterogeneity. A detailed profiling of the protein–lipid contacts with tyrosine, tryptophan, lysine and arginine residues shows increased non-polar contacts in the cytoplasmic leaflet for both PFTs, with a higher number of atomic contacts in the case of AHL in the extracellular leaflet due to the mushroom-like topology of the pore complex. The short ranged nature of the perturbations observed in this simple one component membrane suggests inherent plasticity of membrane lipids enabling the recovery of the structure and membrane fluidity even in the presence of these large oligomeric transmembrane protein assemblies. This observation has implications in membrane repair processes such as budding or vesicle fusion events used to mitigate PFT virulence, where the underlying lipid dynamics and fluidity in the vicinity of the pore complex are expected to play an important role.


 Please wait while we load your content...
Please wait while we load your content...