Formation of peptide-based oligomers in dimethylsulfoxide: identifying the precursor of fibril formation†
Abstract
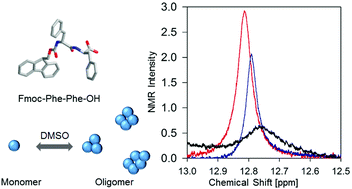
The well-studied dipeptide fluorenylmethyloxycarbonyl-di-phenylalanine (FmocFF) forms a rigid hydrogel upon dissolving in dimethylsulfoxide (DMSO) and dilution in H2O. Here, we explored the pre-aggregation of the peptide in pure DMSO by vibrational spectroscopies, X-ray powder diffraction and dynamic light scattering. Our results show an equilibrium between a dominant population of amorphous oligomers (on a length scale of 2 nm) and a small number of protofibrils/fibrils (on a length scale of 30 nm in the centimolar and of 200 nm in the sub-molar region). To probe the mechanism underlying the formation of these protofilaments, we measured the 1H-NMR, IR and visible Raman spectra of DMSO containing different FmocFF concentrations, ranging between 10 and 300 mM. Our data reveal that interpeptide hydrogen bonding leads to the self-assembly of FmocFF in the centimolar region, while π–π stacking between Fmoc-groups is observed above 100 mM. The high 3J(HNHCα) coupling constant of the N-terminal amide proton indicates that the Fmoc end-cap of the peptide locks the N-terminal residue into a conformational ensemble centered at a ϕ-value of ca. −120°, which corresponds to a parallel β-sheet type conformation. The 3J(HNHCα) coupling constant of the C-terminal residue is indicative of a polyproline II (pPII)/βt mixture. Our results suggest that the gelation of FmocFF caused by the addition of a small amount of water to DMSO mixtures is facilitated by the formation of disordered protofibrils in pure DMSO.


 Please wait while we load your content...
Please wait while we load your content...
