Tough polymeric hydrogels using ion-pair comonomers†
Abstract
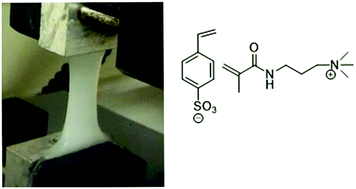
Hydrogels with excellent mechanical properties were synthesized by radical photo-polymerization of three different types of ion-pair comonomers (IPC), without requiring any chemical cross-linking agent. Insoluble gels formed only at a specific solution concentration range, which was unique to the particular salt. The gels changed properties after one day soaking in water, becoming less stiff and more extendible, but remained stable after that. Strains of up to 4000% were measured for one salt pair and ultimate stresses of up to 2.53 MPa for another. Self-healing properties were noted along with some recovery of creep, due to the non-covalent nature of the gel. These properties arise through a combination of electrostatic and hydrophobic interactions of the polymer chains. Immersion of the gels in salt solution screened the electrostatic interactions, resulting in dissolution of the gel.


 Please wait while we load your content...
Please wait while we load your content...