Arrested dynamics in a model peptide hydrogel system†
Abstract
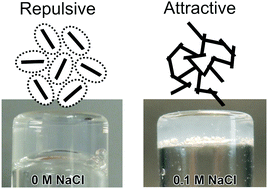
We report here on a peptide hydrogel system, which in contrast to most other such systems, is made up of relatively short fibrillar aggregates, discussing resemblance with colloidal rods. The synthetic model peptides A8K and A10K, where A denotes alanine and K lysine, self-assemble in aqueous solutions into ribbon-like aggregates having an average length 〈L〉 on the order of 100 nm and with a diameter d ≈ 6 nm. The aggregates can be seen as weakly charged rigid rods and they undergo an isotropic to nematic phase transition at higher concentrations. Translational motion perpendicular to the rod axis gets strongly hindered when the concentration is increased above the overlap concentration. Similarly, the rotational motion is hindered, leading to very long stress relaxation times. The peptide self-assembly is driven by hydrophobic interactions and due to a net peptide charge the system is colloidally stable. However, at the same time short range, presumably hydrophobic, attractive interactions appear to affect the rheology of the system. Upon screening the long range electrostatic repulsion, with the addition of salt, the hydrophobic attraction becomes more dominant and we observe a transition from a repulsive glassy state to an attractive gel-state of the rod-like peptide aggregates.
- This article is part of the themed collection: Soft Matter 15th Anniversary Perspectives


 Please wait while we load your content...
Please wait while we load your content...