Scalar activity induced phase separation and liquid–solid transition in a Lennard-Jones system†
Abstract
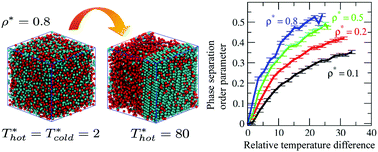
We report scalar activity induced phase separation and crystallization in a system of 3-d Lennard-Jones particles taken at state points spanning from the gas to the liquid regime using molecular dynamics simulation (MD). Scalar activity was introduced by increasing the temperature of half of the particles (labeled ‘hot’) while keeping the temperature of the other half constant at a lower value (labeled ‘cold’). The relative temperature difference between the two subsystems is considered as a measure of the activity. From our simulations we observe that the two species tend to phase separate at sufficiently high activity ratio. The extent of separation is quantified by the defined order parameter and the entropy production during this process is determined by employing the two-phase thermodynamic (2PT) model and the standard modified Benedict–Webb–Rubin (MBWR) equation of state for a LJ fluid. We observe that the extent of the phase separation and entropy production increases with the density of the system. From a cluster analysis, we obtain the mean number of clusters 〈ncl〉, and the mean size of the largest cluster 〈n0〉 in the system, complementing each other. Bond orientation order parameters reveal that the so formed largest cluster also develops solid-like order consisting of both FCC and HCP packing. The presence of such crystalline order is also supported by a common neighbor analysis.


 Please wait while we load your content...
Please wait while we load your content...