Entropy production in thermal phase separation: a kinetic-theory approach
Abstract
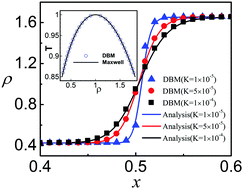
Entropy production during the process of thermal phase-separation of multiphase flows is investigated by means of a discrete Boltzmann kinetic model. The entropy production rate is found to increase during the spinodal decomposition stage and to decrease during the domain growth stage, attaining its maximum at the crossover between the two. Such behaviour provides a natural criterion to identify and discriminate between the two regimes. Furthermore, the effects of heat conductivity, viscosity and surface tension on the entropy production rate are investigated by systematically probing the interplay between non-equilibrium energy and momentum fluxes. It is found that the entropy production rate due to energy fluxes is an increasing function of the Prandtl number, while the momentum fluxes exhibit an opposite trend. On the other hand, both contributions show an increasing trend with surface tension. The present analysis inscribes within the general framework of non-equilibrium thermodynamics and consequently it is expected to be relevant to a broad class of soft-flowing systems far from mechanical and thermal equilibrium.


 Please wait while we load your content...
Please wait while we load your content...