Cation-induced folding of alginate-bearing bilayer gels: an unusual example of spontaneous folding along the long axis
Abstract
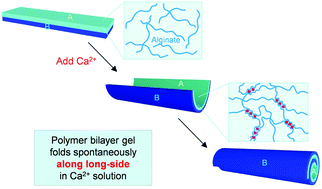
The spontaneous folding of flat gel films into tubes is an interesting example of self-assembly. Typically, a rectangular film folds along its short axis when forming a tube; folding along the long axis has been seen only in rare instances when the film is constrained. Here, we report a case where the same free-swelling gel film folds along either its long or short axis depending on the concentration of a solute. Our gels are sandwiches (bilayers) of two layers: a passive layer of cross-linked N,N′-dimethylyacrylamide (DMAA) and an active layer of cross-linked DMAA that also contains chains of the biopolymer alginate. Multivalent cations like Ca2+ and Cu2+ induce these bilayer gels to fold into tubes. The folding occurs instantly when a flat film of the gel is introduced into a solution of these cations. The likely cause for folding is that the active layer stiffens and shrinks (because the alginate chains in it get cross-linked by the cations) whereas the passive layer is unaffected. The resulting mismatch in swelling degree between the two layers creates internal stresses that drive folding. Cations that are incapable of cross-linking alginate, such as Na+ and Mg2+, do not induce gel folding. Moreover, the striking aspect is the direction of folding. When the Ca2+ concentration is high (100 mM or higher), the gels fold along their long axis, whereas when the Ca2+ concentration is low (40 to 80 mM), the gels fold along their short axis. We hypothesize that the folding axis is dictated by the inhomogeneous nature of alginate-cation cross-linking, i.e., that the edges get cross-linked before the faces of the gel. At high Ca2+ concentration, the stiffer edges constrain the folding; in turn, the gel folds such that the longer edges are deformed less, which explains the folding along the long axis. At low Ca2+ concentration, the edges and the faces of the gel are more similar in their degree of cross-linking; therefore, the gel folds along its short axis. An analogy can be made to natural structures (such as leaves and seed pods) where stiff elements provide the directionality for folding.


 Please wait while we load your content...
Please wait while we load your content...
