Sonocrystallization of poly(3-hexylthiophene) in a marginal solvent†
Abstract
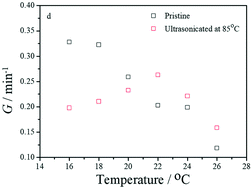
The application of ultrasonication to P3HT in anisole can dramatically affect the crystallization of P3HT. The ultrasonication conditions were modulated by varying the ultrasonication time, ultrasonication power and ultrasonication temperature. Ultrasonicating at the dissolution temperature (85 °C) causes the concentration of the P3HT solution to fluctuate. When fixing the ultrasonication power at 100 W and ultrasonication time at 3 min, for P3HT crystallized in solution at 16 °C, the crystallization kinetics of ultrasonicated P3HT is slower than that of pristine P3HT. The nanofiber aggregation density and crystallinity of ultrasonicated P3HT are lower than those of pristine P3HT, and the nanofiber aggregation size is larger. For P3HT crystallized in solution at 20 °C, the crystallization kinetics, nanofiber morphology and crystallinity of ultrasonicated P3HT are similar to those of pristine P3HT. For P3HT crystallized in solution at 26 °C, the crystallization kinetics of ultrasonicated P3HT is faster than that of pristine P3HT, the nanofiber aggregation size is larger, and the crystallinity is higher. Fixing the crystallization temperature at 16 °C and varying the ultrasonication time and ultrasonication power can effectively modulate the crystallization kinetics of P3HT. When the P3HT solution is ultrasonicated at the crystallization temperature (16 °C), in addition to fluctuations in the concentration, ultrasonication promotes the disentanglement of P3HT chains. The combination of the two effects of ultrasonication is more beneficial for the crystallization of P3HT when solvophobic forces exist in a marginal solvent.


 Please wait while we load your content...
Please wait while we load your content...