The inter-micellar interaction enthalpies of DTAB/TX100 mixed micelles and their structural transitions†
Abstract
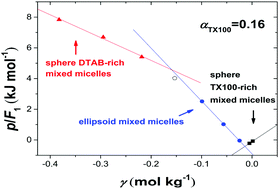
The heats of mixing for a series of DTAB/TX100 mixed surfactant aqueous solutions were measured by flow-mixing calorimetry and isothermal titration calorimetry (ITC) at 298.15 K and 85 kPa, which were used to calculate the inter-micellar interaction enthalpies (−ΔHC). The signs of −ΔHC for pure DTAB and TX100 micellar systems were contrary to the inter-micellar interaction parameters reported for the same systems in the literature, suggesting that these interaction parameters might have a Gibbs free energy character dominated by entropy changes. It was found that the inter-micellar interaction enthalpies varied with the total surfactant concentration and the mixed ratio of the two surfactants, and characterized different structures of the mixed micelles. These phenomena were discussed based on the effects of structural water around the micellar interface, the hydration of counterions, and the repulsive Coulombic interaction.


 Please wait while we load your content...
Please wait while we load your content...