Interconnected macroporous 3D scaffolds templated from gelatin nanoparticle-stabilized high internal phase emulsions for biomedical applications
Abstract
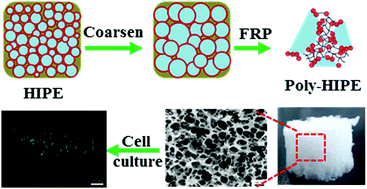
Here we report on the successful preparation of open-cellular macroporous 3D scaffolds templated from gelatin nanoparticle-stabilized HIPEs with acrylamide (AM) as the monomer in the continuous phase. Tuning the gelatin nanoparticle concentration or AM content led to different porous structures with void diameters varying between 30 and 78 μm. More importantly, keeping HIPEs at room temperature to undergo a limited kinetic coarsening before polymerization could greatly improve the interconnectivity and pore size of the scaffolds, with the average diameters (approx. 118 μm) being enlarged 1.5-fold. Additionally, the scaffolds had a character of soft tissue with compressive modulus more than 150 kPa. The cell culture assay confirmed that HepG2 cells not only could adsorb on but also were grown inside the scaffolds, representing a characteristic of the good biocompatibility of the scaffolds. Our work suggests that the 3D scaffolds fabricated from gelatin nanoparticle-stabilized HIPE templates are promising culture substrates for a wide range of applications in the biomedical field.


 Please wait while we load your content...
Please wait while we load your content...