Selective protein recognition in supported lipid bilayer arrays by tailored, dual-mode deep cavitand hosts†
Abstract
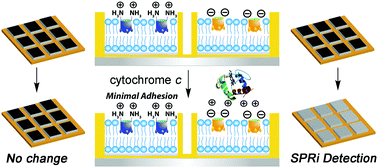
Self-folding deep cavitands with variably functionalized upper rims are able to selectively immobilize proteins at a biomimetic supported lipid bilayer surface. The immobilization process takes advantage of the dual-mode binding capabilities of the hosts, combining a defined binding pocket with upper rim charged/H-bonding groups. A variety of proteins can be selectively immobilized at the bilayer interface, either via complementary charge/H-bonding interactions, cavity-based molecular recognition, or a combination of both. The immobilization process can be used to bind unmodified native proteins, epitopes for bioadhesion, or proteins covalently modified with suitable RNMe3+ binding “handles” and charged groups that can either match or mismatch with the cavitand rim. The immobilization process can be monitored in real time using surface plasmon resonance (SPR) spectroscopy, and applied to the construction of cavitand:lipid arrays using the hosts and trehalose vitrified phospholipid vesicles. The selective, dual-mode protein recognition is maintained in the arrays, and can be visualized using SPR imaging.


 Please wait while we load your content...
Please wait while we load your content...