Aggregation and deposition of in situ formed colloidal particles in the presence of polyelectrolytes†
Abstract
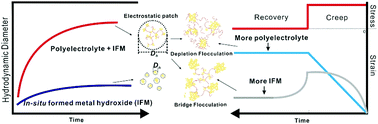
In this work, aggregation and deposition of in situ formed magnesium hydroxide (IFM) in the presence of hydrolyzed polyacrylamide (HPAM) were investigated. Relative concentrations of interactants, as well as other experimental conditions, were changed to elucidate the interaction mechanisms from microscopic to macroscopic levels. Light scattering measurements were used to investigate the aggregation kinetics, fractal dimension, and collision efficiency of the aggregates on a microscopic level. Electrophoretic mobility and TEM were utilized to measure the charging properties and morphologies of aggregates, respectively. Adsorption and rheology experiments were performed to determine the deposition mechanism at higher concentrations of interactants on a macroscopic level. The results demonstrate that the initial rapid aggregation of IFM in the presence of HPAM is due to an electrostatic patch mechanism. In addition, the deposition was accelerated by flocculation with different mechanisms. When more IFM is involved, bridging flocculation dominates; when more HPAM is added, depletion flocculation plays a leading role. The results of this work may provide further insight into understanding the aggregation and deposition of in situ formed natural/engineered particles in the presence of oppositely charged polyelectrolytes, as well as provide new possibilities for produced water treatment, biomedical applications, biomineralization, etc.

 Please wait while we load your content...
Please wait while we load your content...