Early stage kinetics of polyelectrolyte complex coacervation monitored through stopped-flow light scattering†
Abstract
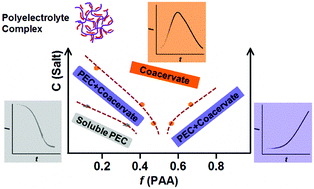
Polyelectrolyte complexes (PECs) between poly(acrylic acid) (PAA) and poly(diallyldimethylammonium chloride) (PDADMAC), a model system forming coacervate particles via electrostatic interaction at pH 10, were prepared by a stopped-flow (SF) fast mixing technique at different mixing charge ratios (z) and ionic strengths. Both PEC final morphologies prepared by either SF or manual one-shot mixing are similar at bench time. In situ light scattering combined with the SF technique pointed-out, however, the presence of three distinct early stage kinetic behaviors in the formation of PECs. The first stage observed at low z is ascribed to the relaxation/reorganization of soluble PECs. At higher z or in the presence of salt, a second stage is found corresponding to the aggregation and/or rearrangement of small soluble PECs into larger structures. Redistribution of excess charges among those PECs produces some neutral condensed coacervate droplets as well, coexisting with PECs in a wide range of mixing ratios. Finally, a last process featured with bell-shaped curves indicates the full coacervation that quickens while approaching charge neutrality and/or at higher ionic strength.


 Please wait while we load your content...
Please wait while we load your content...