Supramolecular control over the structural organization of a second-order NLO-active organogelator†
Abstract
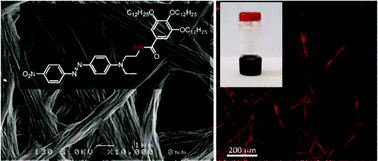
A study of the structural parameters which govern the supramolecular organization of an organogelator built from the Disperse Red moiety is proposed. In particular, the key balance between intermolecular H-bonding and/or π–π interactions is addressed by comparing the effect of a secondary amide vs. an ester linker within the molecular structure. Solution 1H-NMR studies show the superiority of the former interaction in promoting the nanostructuring process, allowing it to reach a gel state in toluene. The nanostructures obtained from both the amide and the ester derivatives were also studied in the solid state. In particular, the use of second-harmonic generation microscopy demonstrates that an anisotropic organization of the material can even be observed in the case of the ester derivative, which demonstrates the efficiency of the tris(alkoxy)benzene unit in directing the self-assembly process, independently of additional H-bond interactions.


 Please wait while we load your content...
Please wait while we load your content...