T-shaped monopyridazinotetrathiafulvalene-amino acid diad based chiral organogels with aggregation-induced fluorescence emission†
Abstract
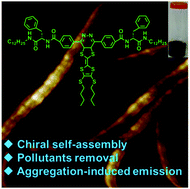
A series of pyridazine coupled tetrathiafulvalene T-shaped derivatives with varying amino acid moieties have been synthesized and their gelation properties were studied in various organic solvents. Among these derivatives, two gelators bearing glycine or phenylalanine units display efficient gelation in aromatic and polar solvents. Interestingly, these gelators, except for the gelator containing two tryptophan units, are able to gel DMF via a solution-to-gel transformation when triggered with sonication for less than 20 s or cooled below zero. A number of experiments revealed that these gelator molecules self-assembled into elastically interpenetrating three-dimensional chiral fibrillar aggregates. Importantly, all of the resulting gels result in a dramatic enhancement of the fluorescence intensity compared with their hot solution in spite of the absence of a conventional fluorophore unit and the fluorescence was effectively quenched by the introduction of C60. Moreover, the gelators can be utilized for the removal of different types of toxic molecules, such as aromatic solvents and cationic dyes, from wastewater.
 Please wait while we load your content...
Please wait while we load your content...