Coacervation and precipitation in polysaccharide–protein systems†
Abstract
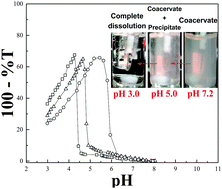
Precipitation poses a consistent problem for the growing applications of biopolymer coacervation, but the relationship between the two types of phase separation is not well understood. To clarify this relationship, we studied phase separation as a function of pH and ionic strength, in three systems of proteins with anionic polysaccharides: β-lactoglobulin (BLG)/hyaluronic acid (HA); BLG/tragacanthin (TG); and monoclonal antibody (mAb)/HA. We found that coacervation and precipitation are intrinsically different phenomena, responsive to different factors, but their simultaneity (for example with changing pH) may be confused with transitions from one state to another. We propose that coacervate does not literally turn into precipitate, but rather that both coacervate and precipitate are in equilibrium with free protein and polyanion, so that dissolution of one and formation of the other can overlap in time. While protein–polyanion complexes must achieve neutrality for coacervation, precipitation only requires tight binding which leads to the expulsion of counterions and water molecules. The pH-dependence of phase separation, considered in terms of protein and polyion charge, revealed that the electrostatic magnitude of the protein's polymer-binding site (“charge patch”) plays a key role in the strength of interaction. These findings were supported by the inhibition of precipitation, seen when the bulky side chains of TG impede close protein–polymer interactions.

 Please wait while we load your content...
Please wait while we load your content...