A hypothetical hierarchical mechanism of the self-assembly of the Escherichia coli RNA polymerase σ70 subunit†
Abstract
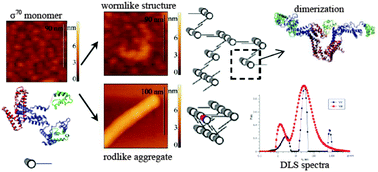
Diverse morphology of aggregates of amyloidogenic proteins has been attracting much attention in the last few years, and there is still no complete understanding of the relationships between various types of aggregates. In this work, we propose the model, which universally explains the formation of morphologically different (wormlike and rodlike) aggregates on the example of a σ70 subunit of RNA polymerase, which has been recently shown to form amyloid fibrils. Aggregates were studied using AFM in solution and depolarized dynamic light scattering. The obtained results demonstrate comparably low Young's moduli of the wormlike structures (7.8–12.3 MPa) indicating less structured aggregation of monomeric proteins than that typical for β-sheet formation. To shed light on the molecular interaction of the protein during the aggregation, early stages of fibrillization of the σ70 subunit were modeled using all-atom molecular dynamics. Simulations have shown that the σ70 subunit is able to form quasi-symmetric extended dimers, which may further interact with each other and grow linearly. The proposed general model explains different pathways of σ70 subunit aggregation and may be valid for other amyloid proteins.

 Please wait while we load your content...
Please wait while we load your content...