How the cation 1-butyl-3-methylimidazolium impacts the interaction between the entrapped water and the reverse micelle interface created with an ionic liquid-like surfactant†
Abstract
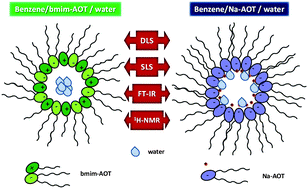
The behavior of the interfacial water entrapped in reverse micelles (RMs) formed by the ionic liquid-like surfactant 1-butyl-3-methylimidazolium 1,4-bis-2-ethylhexylsulfosuccinate (bmim-AOT) dissolved in benzene (or chlorobenzene) was investigated using noninvasive techniques such as dynamic light scattering (DLS), static light scattering (SLS), FT-IR and 1H NMR. The DLS and SLS results reveal the formation of discrete spherical and non-interacting water droplets stabilized by the bmim-AOT surfactant. Moreover, since the droplet size increases as the W0 (W0 = [water]/[surfactant]) value increases, water interacts with the RM interface. From FT-IR and 1H NMR data, a weaker water–surfactant interaction in bmim-AOT RMs in comparison with the RMs created by sodium 1,4-bis-2-ethylhexylsulfosuccinate (Na-AOT) is detected. Consequently, there are less water molecules interacting with the interface in bmim-AOT RMs, and their hydrogen bond network is not completely disrupted as they are in Na-AOT RMs. The results show how the nature of the new cation impacts the interaction between the entrapped water and the RM interface, modifying the interfacial water structure in comparison with the results known for Na-AOT.

 Please wait while we load your content...
Please wait while we load your content...