Formation of polyelectrolyte multilayers: ionic strengths and growth regimes
Abstract
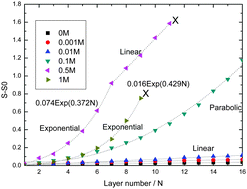
This article presents a study of layer-by-layer (LbL) formation of poly-electrolyte multilayers (PEMs). Upon increasing ionic strength LbL growth patterns vary from linear for the lowest salt concentrations ([NaCl] = 0, 0.001, and 0.01 M) to exponential (for [NaCl] = 0.5 and 1 M). The slope of the linear growth at the lowest ionic strengths increases with increasing [NaCl]. During the LbL process at 0.5 M NaCl we observe a cross over from exponential to linear growth for which the slope is orders of magnitude larger than those observed at low salt concentrations. We provide a comprehensive interpretation of these growth behaviors, which are also reported for many other LbL PEM systems, based on the generic features of the phase diagram of aqueous solutions of mixtures of oppositely charged poly-electrolytes. Processes occurring in LbL formation of PEMs can be understood as moving in the direction of equilibrium, while never achieving it. The experimental model system in this study was: polydiallyldimethylammonium chloride/polystyrene sulfonate (PDADMAC/PSS). PEM formation was followed in situ by optical reflectometry in combination with well-controlled transport conditions (impinging jet stagnation point flow).
- This article is part of the themed collection: Open access articles from Soft Matter

 Please wait while we load your content...
Please wait while we load your content...