Probing the Ca2+-assisted π–π interaction during Ca2+-dependent protein folding†
Abstract
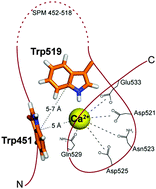
Protein folding is governed by a balance of non-covalent interactions, of which cation–π and π–π play important roles. Theoretical calculations revealed a strong cooperativity between cation–π involving alkali and alkaline earth metal ions and π–π interactions, but however, no experimental evidence was provided in this regard. Here, we characterized a Ca2+-binding self-processing module (SPM), which mediates a highly-specific Ca2+-dependent autocatalytic processing of iron-regulated protein FrpC secreted by the pathogenic Gram-negative bacterium Neisseria meningitidis. The SPM undergoes a Ca2+-induced transition from an intrinsically unstructured conformation to the compact protein fold that is ultimately stabilized by the π–π interaction between two unique tryptophan residues arranged in the T-shaped orientation. Moreover, the pair of tryptophans is located in a close vicinity of a calcium-binding site, suggesting the involvement of a Ca2+-assisted π–π interaction in the stabilization of the tertiary structure of the SPM. This makes the SPM an excellent model for the investigation of the Ca2+-assisted π–π interaction during Ca2+-induced protein folding.

 Please wait while we load your content...
Please wait while we load your content...