Adsorption behaviors of novel betaines on the wettability of the quartz surface
Abstract
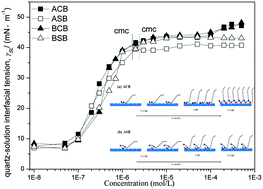
The contact angle measurements for the aqueous solutions of two pairs of zwitterions on quartz surfaces have been investigated by the sessile drop analysis. The different physicochemical parameters such as the critical micelle concentration (CMC), surface tension, contact angle, surface excess on air–liquid and solid–liquid interfaces and work of adhesion have been estimated. The obtained results show that the contact angle of surfactants such as alkyl carboxylbetaine (ACB) and ditolyl substituted alkyl carboxylbetaine (BCB) remains almost constant in a wide range of surfactant concentration and increases gradually above CMC, which are quite different from traditional surfactants reported in the literature. Surfactants with bigger polar groups have a more steric effect on the quartz surface and the contact angle remains relatively unchanged. Moreover, an increase in quartz–liquid interfacial tension (γSL) has been observed due to the adsorption of four zwitterionic surfactants. Especially for ACB and BCB, at the surfactant concentrations higher than 5 × 10−5 mol L−1, a moderate increase in the interfacial tension of the quartz–liquid is observed, which suggests that ACB and BCB can form a saturated adsorption film briefly on the quartz surface and then adsorb again. However, the addition of alkyl sulfobetaine (ASB) and ditolyl substituted alkyl sulfobetaine (BSB) after CMC cannot adsorb on the quartz surface again due to the steric effect of bigger polar groups.

 Please wait while we load your content...
Please wait while we load your content...