Why does high pressure destroy co-non-solvency of PNIPAm in aqueous methanol?
Abstract
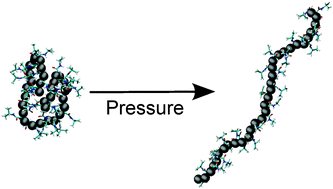
It is well known that poly(N-isopropylacrylamide) (PNIPAm) exhibits an interesting, yet puzzling, phenomenon of co-non-solvency. Co-non-solvency occurs when two competing good solvents for PNIPAm, such as water and alcohol, are mixed together. As a result, the same PNIPAm collapses within intermediate mixing ratios. This complex conformational transition is driven by preferential binding of methanol with PNIPAm. Interestingly, co-non-solvency can be destroyed when applying high hydrostatic pressures. In this work, using a large scale molecular dynamics simulation employing high pressures, we propose a microscopic picture behind the suppression of the co-non-solvency phenomenon. Based on thermodynamic and structural analysis, our results suggest that the preferential binding of methanol with PNIPAm gets partially lost at high pressures, making the background fluid reasonably homogeneous for the polymer. This is consistent with the hypothesis that the co-non-solvency phenomenon is driven by preferential binding and is not based on depletion effects.

 Please wait while we load your content...
Please wait while we load your content...