The influence of ionic strength and mixing ratio on the colloidal stability of PDAC/PSS polyelectrolyte complexes†
Abstract
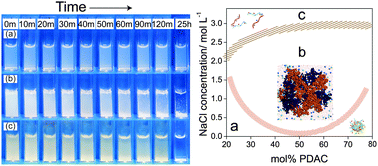
Polyelectrolyte complexes (PECs) form by mixing polycation and polyanion solutions together, and have been explored for a variety of applications. One challenge for PEC processing and application is that under certain conditions the as-formed PECs aggregate and precipitate out of suspension over the course of minutes to days. This aggregation is governed by several factors such as electrostatic repulsion, van der Waals attractions, and hydrophobic interactions. In this work, we explore the boundary between colloidally stable and unstable complexes as it is influenced by polycation/polyanion mixing ratio and ionic strength. The polymers examined are poly(diallyldimethylammonium chloride) (PDAC) and poly(sodium 4-styrenesulfonate) (PSS). Physical properties such as turbidity, hydrodynamic size, and zeta potential are investigated upon complex formation. We also perform detailed molecular dynamics simulations to examine the structure and effective charge distribution of the PECs at varying mixing ratios and salt concentrations to support the experimental findings. The results suggest that the colloidally stable/unstable boundary possibly marks the screening effects from added salt, resulting in weakly charged complexes that aggregate. At higher salt concentrations, the complexes initially form and then gradually dissolve into solution.

 Please wait while we load your content...
Please wait while we load your content...