Multi-responsible chameleon molecule with chiral naphthyl and azobenzene moieties†
Abstract
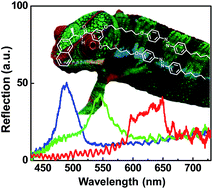
A photochromic chiral molecule with azobenzene mesogens and a (R)-configuration naphthyl moiety (abbreviated as NCA2M) was specifically designed and synthesized for the demonstration of chameleon-like color changes responding to multitudinous external stimuli, such as temperature, light and electric field. The basic phase transition behaviors of NCA2M were first studied by the combination of differential scanning calorimetry (DSC) and polarized optical microscopy (POM). Based on the structure-sensitive X-ray diffraction results obtained at different temperatures, it was comprehended that the NCA2M molecule exhibited the tilted version of highly ordered smectic crystal phase with 5.45 nm layer thickness. Chiral nematic (N*) liquid crystals (LC) with helical superstructures were formed by doping the NCA2M photochromic chiral molecule in an achiral nematic (N) LC medium. By controlling the helical pitch length of N*-LC with respect to temperature, light and electric field, the wavelength of selectively reflected light from the N* photonic crystal was finely tuned. The light-induced color change of N*-LC film was the most efficient method for covering the whole visible region from blue to green and to red, which allowed us to fabricate remote-controllable photo-responsive devices.

 Please wait while we load your content...
Please wait while we load your content...