Anionic deep cavitands enable the adhesion of unmodified proteins at a membrane bilayer†
Abstract
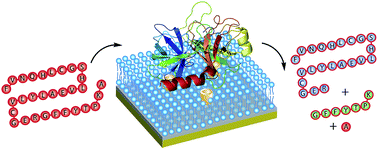
An anionic self-folding deep cavitand is capable of immobilizing unmodified proteins and enzymes at a supported lipid bilayer interface, providing a simple, soft bioreactive surface that allows enzymatic function under mild conditions. The adhesion is based on complementary charge interactions, and the hosts are capable of binding enzymes such as trypsin at the bilayer interface: the catalytic activity is retained upon adhesion, allowing selective reactions to be performed at the membrane surface.


 Please wait while we load your content...
Please wait while we load your content...