Solvent induced helical aggregation in the self-assembly of cholesterol tailed platinum complexes†
Abstract
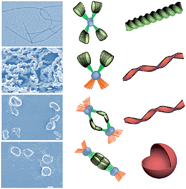
Three alkynylplatinum(II) bipyridyl complexes in which two cholesterol groups are combined with a bipyridyl group via alkyl chains and amido bonds were designed and synthesized. The complexes have different lengths of ethylene glycol chains at the para-position of 1-phenylethyne. All three complexes can self-assemble to gel networks in DMSO, while only the morphology of 1a without an ether chain shows a well-defined right-handed helical structure in layer packing mode. However, 1c with long ethylene glycol chains forms perfect regular left-handed helical structures in aqueous ethanol solution while the volume percentage of water is less than 5% (v/v). As the ratio of water increases, the chirality changes from a left-handed helix to a right-handed helix and the packing mode alters from a monolayer structure to a hexagonal structure. As the ratio of water further increases to greater than 50% (v/v), the structure of the assembly finally transforms into bilayer vesicles. The process of the morphology transition is traced by circular dichroism spectra, powder X-ray diffraction, SEM and TEM images. The result indicates that a polar solvent (water) acts as a trigger to change the self-assembly of the chiral structures of the complex due to the strong hydrophobic interaction between cholesterol groups and the balance of the hydrophobicity and hydrophilicity of the solvent environment.

 Please wait while we load your content...
Please wait while we load your content...