Hydrogels by supramolecular crosslinking of terpyridine end group functionalized 8-arm poly(ethylene glycol)†
Abstract
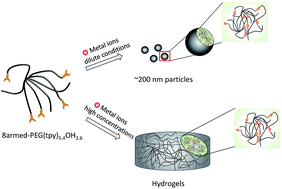
Metallo supramolecular assemblies of an 8-arm poly(ethylene glycol) partially substituted with terpyridyl end-groups and the transition metal ions Ni2+, Fe2+, Co2+ and Zn2+ were studied for their nano-particle formation at dilute conditions and gelation at higher concentrations. The large differences in dissociation rate constants of the metal ligand complexes largely determine the assembly behavior. Thermodynamically stable complexes are generated with Ni2+ and Fe2+ chlorides, which lead to distinct particle sizes of ∼200 nm in dilute conditions. The Co2+ and Zn2+ chlorides provide multiple size distributions revealing that mono and bis-complexes are present at equilibrium. Upon complexation, terpyridyl groups move to the outer sphere giving aggregates with a charged surface. At polymer concentrations above 5 wt%, crosslinking upon addition of transition metal ions provides hydrogels. Elastic hydrogels were obtained with Ni2+, Fe2+ and Co2+ having storage moduli in excess of 20 kPa, whereas Zn2+ gels are relatively viscous. Only Zn2+ gels show a thermoreversible sol to gel transition at a temperature of 25 °C independent of polymer concentration.

 Please wait while we load your content...
Please wait while we load your content...