Thermo-reversible gelation of rod-coil and coil-rod-coil molecules based on poly(dimethyl siloxane) and perylene imides and self-sorting of the homologous pair†
Abstract
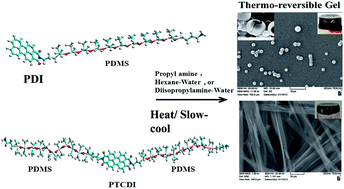
Organogels with perylene derivatives and phthalocyanines reported in the literature so far involve self-assembly promoted by hydrogen bonds, in addition to aromatic and van der Waals interactions. Although the self assembly of these types of molecules without a hydrogen bonding group in the structure occurs in solution or during crystallization, the gelation studies reported so far incorporated a hydrogen bonding pair of the type N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the structure of the molecule. We present a case of thermo-reversible gelation without a hydrogen bonding group in the structure of (1) a coil-rod-coil molecule based on perylenetetracarboxylic diimide (PTCDI) and poly(dimethyl siloxane) (PDMS) and (2) a rod-coil molecule with perylene dicarboxylic imide (PDI) and PDMS. However IR spectroscopy shows the presence of multiple types of hydrogen bonding between the solvents and the gelator molecules. In addition, publications so far on gelation of perylene diimide based molecules involve groups attached to both imide nitrogens and with or without substitution in the bay position. We discuss here the gelation with a Mono-substituted perylene imide. The PDMS segment was attached to one side of PDI (Mono-PDMS) or to both imide nitrogens of PTCDI (Di-PDMS). The Mono-PDMS is an inverse macromolecular surfactant applicable to non-aqueous systems, and the Di-PDMS is a Gemini surfactant. The PDMS segment that we attached to PTCDI here is longer than most substituents used by other authors. These molecules gel propylamine, as well as mixed solvents of hexane–water and diisopropylamine–water. Both hexane and diisopropylamine dissolve Mono-PDMS and Di-PDMS at room temperature and addition of water results in precipitation. However, heating the solution to about 70 °C, adding water (5–15 wt%) and slowly cooling the solution, lead to gelation. The Di-PDMS forms fibers which are not flat but curved as an eaves trough. The Mono-PDMS forms hollow spheres. Although the Mono-PDMS and Di-PDMS are a homologous pair, blends of these do not show molecular intercalation during gelation, but self-sort. The fibers of Di-PDMS based gels encapsulate the spheres of the Mono-PDMS based gels.
C in the structure of the molecule. We present a case of thermo-reversible gelation without a hydrogen bonding group in the structure of (1) a coil-rod-coil molecule based on perylenetetracarboxylic diimide (PTCDI) and poly(dimethyl siloxane) (PDMS) and (2) a rod-coil molecule with perylene dicarboxylic imide (PDI) and PDMS. However IR spectroscopy shows the presence of multiple types of hydrogen bonding between the solvents and the gelator molecules. In addition, publications so far on gelation of perylene diimide based molecules involve groups attached to both imide nitrogens and with or without substitution in the bay position. We discuss here the gelation with a Mono-substituted perylene imide. The PDMS segment was attached to one side of PDI (Mono-PDMS) or to both imide nitrogens of PTCDI (Di-PDMS). The Mono-PDMS is an inverse macromolecular surfactant applicable to non-aqueous systems, and the Di-PDMS is a Gemini surfactant. The PDMS segment that we attached to PTCDI here is longer than most substituents used by other authors. These molecules gel propylamine, as well as mixed solvents of hexane–water and diisopropylamine–water. Both hexane and diisopropylamine dissolve Mono-PDMS and Di-PDMS at room temperature and addition of water results in precipitation. However, heating the solution to about 70 °C, adding water (5–15 wt%) and slowly cooling the solution, lead to gelation. The Di-PDMS forms fibers which are not flat but curved as an eaves trough. The Mono-PDMS forms hollow spheres. Although the Mono-PDMS and Di-PDMS are a homologous pair, blends of these do not show molecular intercalation during gelation, but self-sort. The fibers of Di-PDMS based gels encapsulate the spheres of the Mono-PDMS based gels.

 Please wait while we load your content...
Please wait while we load your content...