Structure–delivery relationships of lysine-based gemini surfactants and their lipoplexes†
Abstract
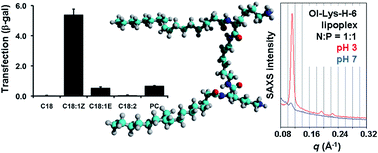
The synthesis and properties of gemini surfactants of the type (R1(CO)-Lys(H)-NH)2(CH2)n are reported. For a spacer length of n = 6, the hydrophobic acyl tail was varied in length (R1 = C8, C10, C12, C14, C16, and C18) and, for R1 = C18, the degree of unsaturation. For R1(CO) = oleoyl (C18:1 Z) the spacer length (n = 2–8) and the stereochemistry of the lysine building block were varied; a ‘half-gemini’ derivative with a single oleoyl tail and head group was also prepared. The potential of the gemini surfactants to transfer polynucleotides across a cell membrane was investigated by transfection of HeLa cells with beta-galactosidase, both in the presence and absence of the helper lipid DOPE. Oleoyl was found to be by far the best hydrophobic tail for this biological activity, whereas the effect of the lysine stereochemistry was less pronounced. The effect of an optimum spacer length (n = 6) was observed only in the absence of helper lipid. The most active surfactant, i.e. the one with oleoyl chains and n = 6, formed liposomes with sizes in the range of 60–350 nm, and its lipoplex underwent a transition from a lamellar to a hexagonal morphology upon lowering the pH from 7 to 3.

 Please wait while we load your content...
Please wait while we load your content...