Multistimuli responsive organogels based on a reactive azobenzene gelator†
Abstract
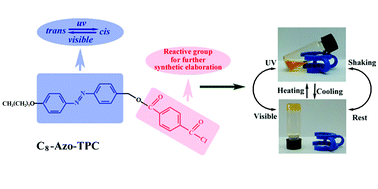
A reactive azobenzene based super organogelator was found to rapidly and reversibly transform a range of hydrophobic solvents from gels to solutions upon changes in temperature, light and shear force. More specifically they formed gels at concentrations as low as 0.08 wt%. Upon heating, exposure to UV light, or application of shear, the π–π bonding was disrupted which resulted in a rapid drop of both modulus and viscosity. This was confirmed by 1H NMR, SAXS, and rheological measurements. Although many examples of organogelators are known in the literature, this is the first time that a reactive group, a benzoyl chloride, has been incorporated in a supramolecular organogel structure. Moreover, this group is available for subsequent synthetic modifications. The presence of benzoyl chloride groups showed a remarkable effect on the formation and properties of the gels. Compared with other approaches, this strategy is advantageous in terms of structural design since it not only produces a multi-responsive soft material but also allows facile modifications which may expand the applications of organogels to other fields.

 Please wait while we load your content...
Please wait while we load your content...