Drug promiscuity of P-glycoprotein and its mechanism of interaction with paclitaxel and doxorubicin†
Abstract
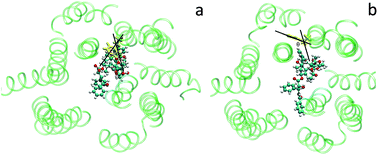
P-glycoprotein (P-gp) pumps a broad range of structurally diverse anti-cancer drugs out of cancer cells. Therefore, multi-drug resistance (MDR) in chemotherapy closely correlates with P-gp. However, how this single transport system recognizes different substrates remains unclear. In this study, we attempt to uncover the mechanism of substrate promiscuity of P-gp by atomistic molecular dynamics simulations. Results indicate that different drugs like paclitaxel and doxorubicin approach the putative binding site of P-gp, and the inner residues are found to be important in this process. An obstacle-overcoming process was observed, illustrating that the inner residues are flexible. Interaction energy calculations suggest that the inner residues possess high affinity toward substrates. The cavity of adaptability to accommodate different drugs would help explain why P-gp has so many different substrates.

 Please wait while we load your content...
Please wait while we load your content...