Tetraalkylammonium ion induced micelle-to-vesicle transition in aqueous sodium dioctylsulfosuccinate solutions†
Abstract
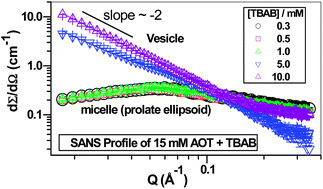
The aggregation behavior of sodium dioctylsulfosuccinate (AOT) in aqueous media containing tetraalkylammonium bromide (TAAB, where alkyl = ethyl (TEAB), propyl (TPAB) and butyl (TBAB)) was studied by surface tension, fluorescence (with pyrene as the probe), small-angle neutron scattering (SANS) and dynamic light scattering (DLS) measurements. A comparison of the critical micelle concentration (cmc) values of AOT in the presence of the salts showed the order TBAB < TPAB < TEAB < NH4Cl < NaCl. Synergism in the cmc occurs when the solution contains a mixture of sodium and tetraalkylammonium counterions. The counterion binding behavior was examined by applying the modified Corrin–Harkins (CH) equation which revealed that a special counterion binding behavior of AOT exists in aqueous solutions with tetraalkylammonium salts. The modified CH equation and DLS data indicate a change in the shape of the surfactant aggregates, which was confirmed by the SANS data. Dehydration of the head group and the counterion during their interaction appears to induce a micelle-to-vesicle transition in the aggregates.

 Please wait while we load your content...
Please wait while we load your content...