Disulfide bond-stabilized physical gels of an asymmetric collagen-inspired telechelic protein polymer†
Abstract
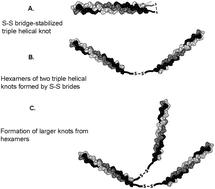
We designed and produced an asymmetric collagen-inspired telechelic protein polymer with end blocks that can form triple helices of different thermal stabilities. Both end blocks consist of a motif that can form triple helices at low temperature, but one of these blocks carries an additional cysteine residue at the end. The cysteine residues can form disulfide bridges under oxidizing conditions, leading to dimer formation. This effectively stabilizes the triple helices, resulting in a double melting peak in differential scanning calorimetry: one corresponding to helices without disulfide bridges and one at significantly higher temperature, corresponding to stabilized helices. Under reducing conditions, the disulfide bridges are broken and the molecule behaves similarly to the symmetric variant. We find that these disulfide bridges also lead to an increase of the elastic modulus of the helical polymer network, probably because the number of helices in the system increases and also the disulfide bridges can crosslink different triple helical nodes.

 Please wait while we load your content...
Please wait while we load your content...