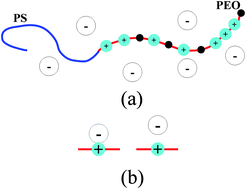
We develop a self-consistent field theory for salt-doped diblock copolymers, such as polyethylene oxide (PEO)–polystyrene with added lithium salts. We account for the inhomogeneous distribution of Li+ ions bound to the ion-dissolving block, the preferential solvation energy of anions in the different block domains, the translational entropy of anions, the ion-pair equilibrium between polymer-bound Li+ and anion, and changes in the χ parameter due to the bound ions. We show that the preferential solvation energy of anions provides a large driving force for microphase separation. Our theory is able to explain many features observed in experiments, particularly the systematic dependence in the effective χ-parameter on the radius of the anions, the observed linear dependence in the effective χ on salt concentration, and increase in the domain spacing of the lamellar phase due to the addition of lithium salts. We also examine the relationship between two definitions of the effective χ parameter, one based on the domain spacing of the ordered phase and the other based on the structure factor in the disordered phase. We argue that the latter is a more fundamental measure of the effective interaction between the two blocks. We show that the ion distribution and the electrostatic potential profile depend strongly on the dielectric contrast between the two blocks and on the ability of the Li+ to redistribute along the backbone of the ion-dissolving block.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...