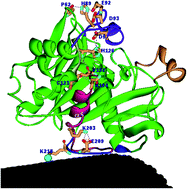
Lipases have been widely used as biocatalysts for numerous biotechnological applications. In an earlier paper, we presented experimental results showing significantly enhanced activity of the Lip2 lipase immobilized on carbon nanotubes (CNTs). In this work, to elucidate the activation mechanism of the lipase, we present an atomic resolution analysis of the lid domain motion of the Lip2 lipase upon immobilization by simulating the protein dynamics. The Lip2 lipase interacts with the CNT at a local site. However, the effects of that interaction are propagated to a remote lid subdomain. The chain of events initiated by the interaction provide the basis for a fundamental understanding of the lid opening mechanism. We identify the structural path followed by co-operative interactions that originate at the interaction site. In addition, salt bridge and hydrogen bonding interactions, which contribute to the propagation of interaction effects to distal regions, are also identified. The present study provides a plausible molecular model for understanding the mechanisms of lid opening of the immobilized lipase. The importance of the structural characteristics of the lipase upon immobilization on the CNT suggests that enzymes may be engineered to be site-specifically immobilized onto a hydrophobic support, such as carbon nanotubes, to achieve enhanced enzymatic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...