Bovine serum albumin unfolds in Couette flow†
Abstract
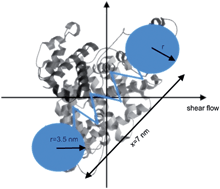
Direct measurements of the effect of hydrodynamic forces generated in Couette flow on the secondary and tertiary structure of bovine serum albumin (BSA) are reported. Real time measurements were made using both fluorescence and

 Please wait while we load your content...
Please wait while we load your content...