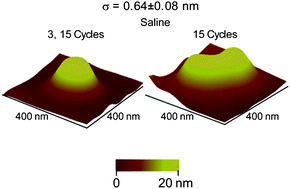
Nanobubbles are believed to have surface charges at the gas–liquid interface. However, the effect of various factors on the formation mechanism and stability of these charged species is not well understood. Using atomic force microscopy (AFM) in tapping mode (TMAFM), the propensity of formation and geometrical distribution of nanobubbles on an ultra-thin polystyrene (PS) film immersed in deionized (DI) water and saline (sodium chloride) solution was investigated. The results reveal that in saline solution, nanobubbles form in larger number and size as compared to nanobubbles in DI water. This is attributed to the enhanced surface charge stabilization of the nanobubbles caused by the electrolyte ions in saline solution. A PS film of higher roughness causes the formation of larger nanobubbles due to the presence of more nucleation sites provided by surface asperities, causing numerous nanobubbles in close proximity to coalesce to form larger ones. The study of the effect of pH shows that nanobubbles are more stable in an alkaline solution than in an acidic solution. The results further reveal that the size of nanobubbles increases with increasing positive substrate bias, whereas there is no measurable change in size with increasing negative substrate bias, possibly due to differential charging at the interfaces across the non-conductive PS film.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...