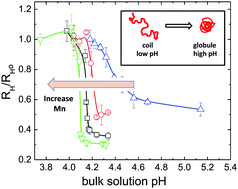
The nature of the conformation transition of a hydrophobic weak polyelectrolyte (PE) chain is critically determined by local electrostatic environment in vicinity of the PE backbone. In this work, we examine the coil-to-globule conformation transition (CGT) and electric potential of a model weak PE, poly(2-vinyl pyridine) (P2VP) of varied molecular weight (Mn), in aqueous solutions at a single molecule level by fluorescence correlation spectroscopy (FCS) and photon counts histogram (PCH). By using FCS, the CGT of P2VP in aqueous solutions is observed upon increasing solution pH, yet showing a strong Mn dependence. The observed CGT is gradual and continuous at low Mn, in sharp contrast to an abrupt, discontinuous one at high Mn. Measured critical transition pH, pHCGT, to induce CGT is found to decrease as increasing Mn. To further elucidate the effect of Mn on CGT and local electrostatic interaction, PCH is employed with pH-sensitive fluorophore probes attached to a P2VP chain to determine the local pH, i.e. the local proton concentration immediately adjacent to an expanded P2VP coil. The electric potential, φ for a single P2VP chain is thereby estimated from the difference between local and bulk pHs, exhibiting a scaling with P2VP polymerization degree, N as φ ∼ N0.1. The increase in φ with increasing Mn suggests an enhanced electrostatic repulsion between local protons and a longer P2VP backbone, leading to the lowering of pHCGT.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...